Probably the first important thing to know is what the difference is between a proton and an electron. Well, for starters, protons have a positive charge, while electrons have a negative charge. Protons are found in the center of an atom, along with neutrons which have no charge, while electrons surround the nucleus. A proton and an electron have a charge of the exact same size, only one is negative and one is positive. Have you ever heard the saying "opposites attract?" Well, when it comes to protons and electrons, the saying is true.

When you hear someone talking about the number of protons in a nucleus, that person is talking about the atomic number. The atomic number can be used to determine the number of neutrons in an atom, along with the atomic mass number. When you subtract the atomic number from the atomic mass number, you get the number of neutrons in an atom! Pretty simple, right? The atomic number is also used to distinguish an element. This brings me to the next important thing to know about chemistry. An element is a pure chemical substance made entirely of one type of atom. For example, every element contains atoms containing a certain amount of protons and electrons. If you change the number of protons an atom has, you completely change the type of element it is. There are about 118 elements total. You can find these elements on a periodic table.

Elements can be classified as a metal, nonmetal, or a metalloid. Each element is represented by letter symbols. For example, the letter symbol for carbon is C, and the symbol for lead is Pb. One last thing to know about elements is that they can't be broken down by chemical reactions.
A compound, however, can be broken down by chemical reactions. Compounds are, obviously, things that are composed of more than one element, a mixture. They contains atoms of different elements combined together in a fixed ration arranged a certain way through chemical bonds. There is an endless list of compounds. Each is represented with a formula. You can classify a compound as ionic or covalent.

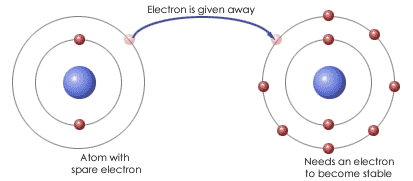
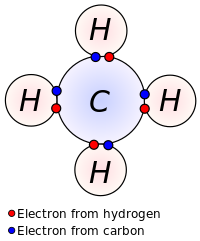
Covalent and ionic bonds are formed in compounds and they are the only types of atomic bonds. A covalent bond is formed between two nonmetals when they have similar electronegativities. Neither nonmetal atom is strong enough to attract electrons from the other; therefore, for stabilization, they share their electrons with others. These bonds have a low polarity and a definite shape. An ionic bond is formed between one metal and one nonmetal. Nonmetal are stronger than metals and can attract electrons easily from metals. Just like protons and electrons, they attract because "opposites attract." Ionic bonds have a high polarity and no definite shape.
These are just some of the basic things to know about chemistry. There is so much more to be learned. In my case, there is so much more to be re-learned. So I guess it's time for me to get studying!
No comments:
Post a Comment